CHEMICAL BONDS
A Chemical bond is an attraction between atoms. This attraction may be seen as the result of different behaviours of the outermost electrons of atoms. Although all of these behaviours merge into each other seamlessly in various bonding situations so that there is no clear line to be drawn between them, nevertheless behaviours of atoms become so qualitatively different as the character of the bond changes quantitatively, that it remains useful and customary to differentiate between the bonds that cause these different properties of condensed matter.
'Covalent Bond' : one or more electrons (often a pair of electrons) are drawn into the space between the two atomic nuclei.
Here, the negatively charged electrons are attracted to the positive charges of both nuclei, instead of just their own. This overcomes the repulsion between the two positively charged nuclei of the two atoms, and so this overwhelming attraction holds the two nuclei in a fixed configuration of equilibrium, even though they will still vibrate at equilibrium position.
Thus, covalent bonding involves sharing of electrons in which the positively charged nuclei of two or more atoms simultaneously attract the negatively charged electrons that are being shared between them.
These bonds exist between two particular identifiable atoms, and have a direction in space, allowing them to be shown as single connecting lines between atoms in drawing , or modelled as sticks between sphere in models. In a polar covalent bonds, one or more electrons are unequally shared between two nuclei.
Covalent bonds often result in the formation of small collection of better -connected atoms called molecules, which in solids and liquids are bound to other molecules by forces that are often much weaker than the covalent bonds that hold the molecules internally together.
Such weak intermolecular bonds give organic molecular substances, such as waxes and oils, their soft bulk character, and their low melting points (in liquids, molecules must cease most structured or oriented contact with each other).
When covalent bonds link long chains of atoms in large molecules, however (as in polymers such as nylon), or when covalent bonds extend in networks though solids that are not composed of discrete molecules (such as diamond or quartz or the silicate minerals in many types of rock) then the structures that result may be both strong and tough, at least in the direction oriented correctly with networks of covalent bonds. Also, the melting points of such covalent polymers and networks increase greatly.
Ionic bonds: the bonding electron is not shared at all, but transfered . In this type of bond, the outer atomic orbital of one atom has a vacancy which allows addition of one or more electrons. These newly added electrons potentially occupy a lower energy-state (effectively closer to more nuclear charge) than they experience in different atom.
Thus, one nucleus offers a more tightly bound position to an electron than does another nucleus, with the result that one atom may transfer an electron to the other.
This transfer causes one atom to assume a net positive charge, and the other to assume a net negative charge. The bond then result from electrostatic attraction between atoms, and the atoms become positive or negatively charged ions.
Ionic bonds may be seen as extreme examples of polarization in covalent bonds. often, such bonds have no particular orientation in space, since they result from equal electrostatic attraction of each ion to all ions around them.
Ionic Bonds are strong (and thus ionic substances require high temperature to melt) but also brittle, since the forces between ions are short-range, an do not easily bridge cracks and fractures.
This type of bond gives a characteristic physical character to crystals of classic minerals salts, such as table salt.
Metallic Bond:
In this type of bonding, each atom in a metal donates one or more electrons to a "Sea" of electrons that reside between many metals atoms. In this sea, each electron is free (by virtue of its wave nature) to be associated with a great many atoms at once.
The bond results because the metal atoms become somewhat positively charged due to loss of their electrons, while the electrons remain attracted to many atoms, without being part of any given atom.
Metallic Bonding may be seen as an extreme example of delocalization of electrons over a large system of covalent bonds, in which every atom participates. This type of bonding is often very strong (resulting in the tensile strength of metals).
However, metallic bonds are more collective in nature than other types, and so they allow metal crystals to more easily deform, because they are composed of atoms attracted to each other, but not in any particularly-oriented ways.
This results in the malleability of metals. The sea of electrons in metallic bonds causes the characteristically good electrical and thermal conductivity of metals, and also their "shiny" reflection of most frequencies of white light.
Aromatic (aryl) compounds:
- An aromatic (or aryl) compound contains a set of covalently bound atoms with specific characteristics
- A delocalized conjugated π system, most commonly an arrangement of alternating single and double bonds.
- Coplanar structure, with all the contributing atoms in the same plane
- contributing atoms arranged in one or more rings.
- A number of π delocalized electrons that is even, but not a multiple of 4.That is, 4n+2 number of π electrons, where n=0,1,2,3, and so on. This is known as Huckel's Rule.
All bonds can be explained by quantum theory, but, in practice, simplification rules allow chemist to predict the strength, directionality, and polarity of bonds.
The octet rule and VSEPR theory are two examples. More sophisticated theories are valence bond theory which includes orbital hybridization an d resonance, and linear combination of atomic orbitals molecular orbital method which includes ligand field theory.
Electrostatics are used to describe bond polarities and the effects they have on chemical substances.
Understanding Biochemistry
There are two common ( and equivalent ) ways to describe molecular mass; both are used. The first is molecular weight , or relative molecular mass, denoted Mr.
The Molecular weight of a substance is defined as the ratio of the mass of a molecule of that substance to one-twelfth the mass of carbon-12(12C).Since Mr is a ratio, it is dimensionless- it has no associated units.
The second is molecular mass, denoted m. This is simply the mass of one molecule, or the molar mass divided by Avogadro's number.
The molecular mass, m, is expressed in daltons(abbreviated as Da). one Daltons is equivalent to one-tweflth the mass of carbon-12; a kilodalton (kDa) is 1000daltons; a megadaltons (MDa) is 1 million daltons.
Consider, for example, a molecule with a mass 100 times that of water. we can say of this molecule either Mr=18,000 or m= 18,000 daltons is incorrect.
Another convenient unit for describing the mass of a single atom or molecule is the atomic mass unit (formerly amu, now commonly denoted u).
One atomic mass unit (1u) is defined as one- twelfth the mass of an atom of carbon-12.Since the experimentally measured mass of an atom of carbon-12 is 1.9926 x 10power 23 g, 1u= 1.6606 x10-24 g, The atomic mass unit is convenient for describing the mass of a peak observed by mass spectrometry.
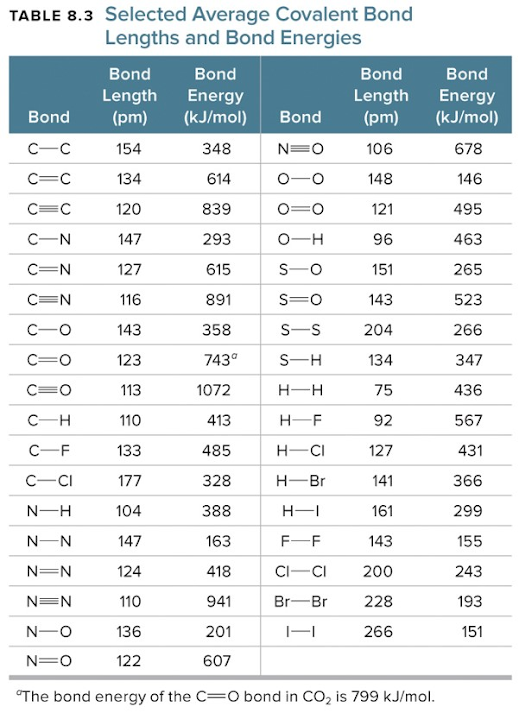
Table-1.3: Typical biond lenghths in pm and bond energies inkj/mol.Bond lengths can be converted to Armstrong by division by 100 (1Armstrong = 100 pm)
figure1.7: Benzene the most widely recognized aromatic compound with six (4n+2,n=1) delocalized electrons